Protocol applications must be submitted through IRBNet.org. IRBNet is secure, web-based, easy to use, and accessible from virtually any computer.
The IRB recommends submitting applications at least six weeks in advance of the anticipated research start date. The IRB receives a high volume of applications, and most need adjustments before they are considered review-ready. Please plan accordingly. Applications requiring full board review must be submitted at least four weeks in advance of the meeting date. The IRB committee meets on the 2nd Tuesday of every month (except when the date falls on a break scheduled on the academic calendar). Exempt and expedited applications are accepted on a rolling basis.
Research with human subjects may not be conducted without the advance approval of the IRB.
Submitting a New Protocol via IRBNet:
- Log in to irbnet.org. If you have not used the system before, you'll first need to register for an account as a new user. Under Research Institution, please select "Lehigh University, Bethlehem, Pennsylvania.”
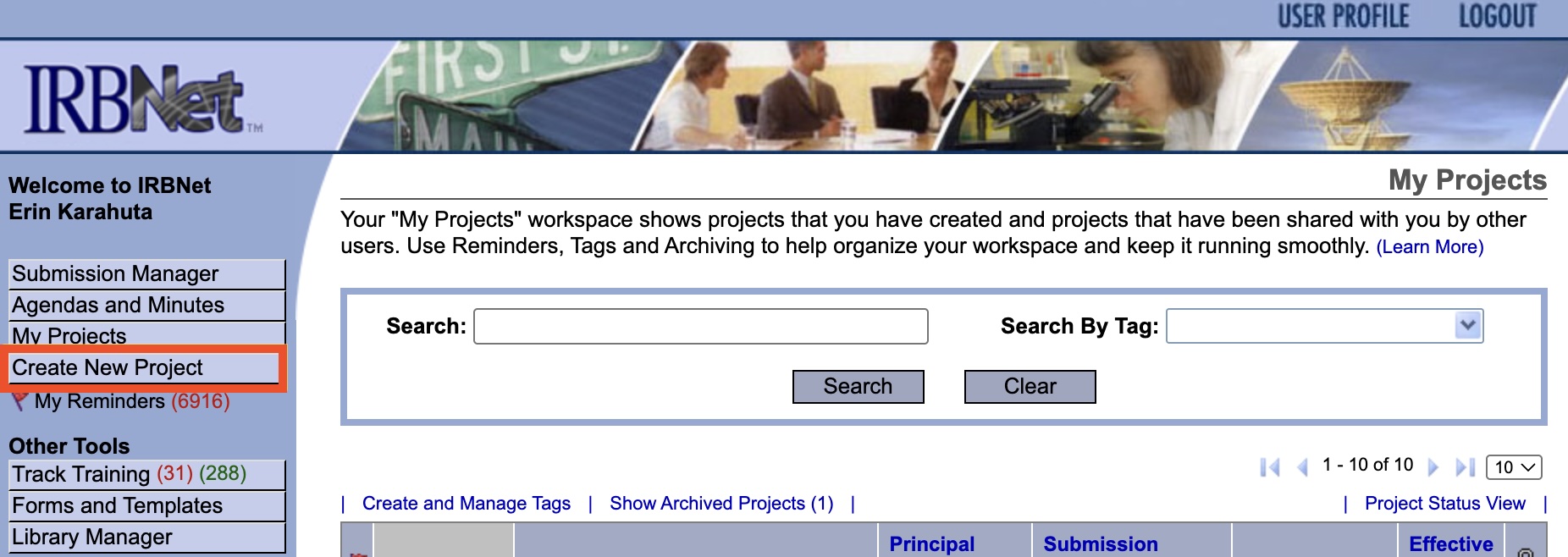
- Click "Create New Project" in the menu on the left-side of your screen.
- Fill in the required information for your study.
- Note: When indicating the Principal Investigator (PI), please review the Policy: Human Subjects Research Principal Investigator. Graduate and undergraduate students are not permitted to serve as PI; students must list their primary faculty advisor as PI.
- Click “Continue.”
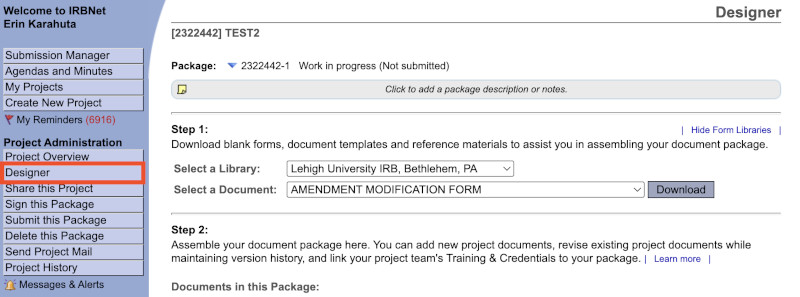
- You are now in the “Designer” window. Here, you can download forms as well as upload documents for IRB review. You can always navigate to the Designer by selecting it from the menu on left-side of your screen:
- To access the Human Subjects Application form, select the “HUMAN SUBJECTS APPLICATION (New Studies)” from the “Select a Document” dropdown menu. Click "Download" to save the application to your local computer.
- Note: the application and all other forms can also be found under "Forms and Templates" in the left-side menu.
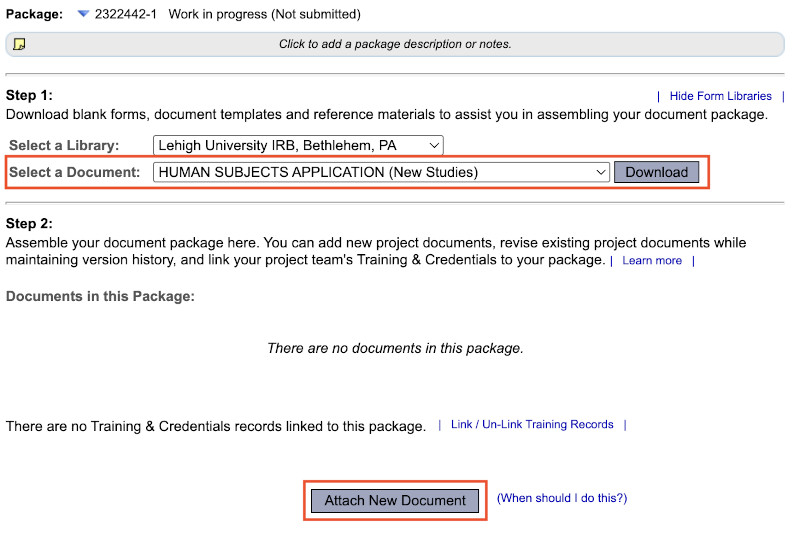
- Once you have completed the application form in full, click "Attach New Document" to upload the completed application form to your submission package. Repeat to add all necessary supplemental documents (e.g. consent form(s), ethics training completion certificates, recruitment materials, survey/interview documents, etc.)
- Click "Share this Project" in the left-side menu to provide access to the submission materials to any faculty and student collaborators. Sharing the project allows these individuals to digitally sign the submission. This is a required step prior to IRB review.
- Note: if your project involves collaboration with academic visitors to Lehigh's campus, you will need to comply with the Collaborating Visitors Policy.
- Click "Sign this Package" in the left-side menu to indicate that all necessary application materials have been uploaded to the IRBNet package. You will be asked to indicate your role at this time. Only faculty eligible to serve as Principal Investigator should select the "Principal Investigator" role. All students and other personnel may choose any other role from the list. All personnel on the IRB protocol must also log in to IRBNet and sign the submission package.
- Once ALL research team members have individually signed the package in IRBNet, click "Submit this Package". Select "Lehigh University IRB" as the reviewing board. Once the package has been submitted, the Office of Research Integrity will conduct a pre-review of your submission materials and notify you if any changes or additional information is necessary prior to IRB review.
Tutorial: New User Registration - registering for IRBNet and affiliating with Lehigh; managing training and credentials.
Tutorial: New Project Submission - designing, sharing, signing, submitting new projects. Managing approval documents and automatic expiration alerts.